The Vector
Volume 7 Issue 8: September 2018
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
Government Relations Chair Message
Breaking Through
Society News
Public Policy
Industry News

Government Relations Chair Message

Dear Colleagues,
I am excited to be serving this year as the chair of ASGCT’s Government Relations Committee, which provides oversight of our strategic priorities in promoting and protecting the interests of our field. Decades of research and development have led us to our current, pivotal point of the field, an amazing time in which we have seen transformative gene therapy approvals in the U.S. This has quickly prioritized not only defining the value of gene therapies for policymakers, but also addressing patient access to that value. As significant contributors to the research and development of the technology that led to these therapies, ASGCT members care deeply about patients’ ability to access approved therapies.
As a result, ASGCT issued a white paper in May—Addressing the Value of Gene Therapy: Enhancing Patient Access to Transformative Treatments—which outlines potential solutions to current barriers to patient access. We will also address this topic at our first Value Summit on September 24 in Washington, DC, which we are calling Advancing Patient Access to the Benefits of Gene Therapy. The event will convene an impressive variety of gene therapy stakeholders – including Congressional staff, CMS staff, payors, and prominent academic and biotechnology leaders – to stimulate discussion and action on ways to both fuel future innovation and enhance patient access.
Another priority for our advocacy program is funding for research, including the basic research that has led to recent crucial clinical applications. For example, federal investment over decades into basic research and the NIH-led Human Genome Project has enabled the fields of gene and cell therapy to flourish today.
ASGCT’s advocacy program informs regulatory policy, as well, through the efforts of the Clinical Trials and Regulatory Affairs (CTRA) Committee. With contributions from the CTRA and Government Relations Committees, ASGCT is holding its first annual liaison meeting with FDA CBER today. The CTRA Committee also submits comments to the FDA on relevant draft guidance documents and provides input to the creation of standards.
These advocacy efforts, among others, elevate the voice of ASGCT members on significant issues affecting the field, to advance the vast potential of gene therapy to alleviate human disease.
Sincerely,
Tim Hunt, JD
ASGCT Government Relations Committee Chair
.jpg)
Breaking Through
A nanostructured lipid carrier for delivery of a replicating viral RNA vaccine provides single, low-dose protection against Zika virus challenge
Summary by: Jesse H. Erasmus1 and Amit P. Khandhar1
1Infectious Disease Research Institute, Seattle, WA, USA
Jesse H. Erasmus, Amit P. Khandhar, Jeff Guderian, Brian Granger, Jacob Archer, Michelle Archer, Emily Gage, Jasmine Fuerte-Stone, Elise Larson, Susan Lin, Ryan Kramer, Rhea N. Coler, Christopher B. Fox, Dan T. Stinchcomb, Steven G. Reed, Neal Van Hoeven. Published: July 15, 2018 DOI: https://doi.org/10.1016/j.ymthe.2018.07.010
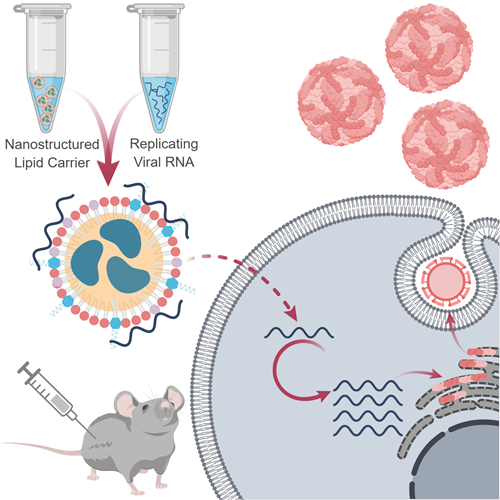 Traditional protein-based vaccine approaches cannot respond rapidly to emerging infectious diseases because the purification step requires extensive optimization and validation for each new vaccine. Nucleic acid vaccine platforms promise to solve this problem by eliminating the reliance on tissue and cell culture and standardizing the production process. The major obstacles faced by nucleic acid-based approaches are stability and delivery. We have developed a platform combining nanoparticle delivery and replicating viral RNAs (rvRNAs). This platform allows RNA and delivery vehicle to be produced separately and mixed at the site of administration, facilitating a rapid and adaptable response to emerging threats.
Traditional protein-based vaccine approaches cannot respond rapidly to emerging infectious diseases because the purification step requires extensive optimization and validation for each new vaccine. Nucleic acid vaccine platforms promise to solve this problem by eliminating the reliance on tissue and cell culture and standardizing the production process. The major obstacles faced by nucleic acid-based approaches are stability and delivery. We have developed a platform combining nanoparticle delivery and replicating viral RNAs (rvRNAs). This platform allows RNA and delivery vehicle to be produced separately and mixed at the site of administration, facilitating a rapid and adaptable response to emerging threats.
The field of messenger RNA (mRNA) delivery has its origins in the early 1990s (Wolff et al. 1990). Unlike gene therapy or any type of DNA delivery, mRNA delivery results in transient cytoplasmic translation of a gene-of-interest and eliminates both the dependence on nuclear translocation and the risks of genome integration. However, the highly susceptible nature of RNA created major translational roadblocks. Novel nanoparticle carrier technologies, with their ability to protect mRNA from enzymatic degradation and deliver it across lipid bilayers, have materially advanced the field. Meanwhile, viral delivery of modified viral genomes, termed replicons, to express heterologous genes was first demonstrated in 1989 (Xiong et al. 1989). These single-stranded positive-sense RNA replicon particles package what is essentially an mRNA molecule that encodes a protein-of-interest and a polyprotein with RNA replicating properties. Like classical non-replicating mRNA approaches, rvRNAs only require cytoplasmic delivery and are short-lived. However, due to their self-amplifying nature and their ability to evade an innate immune response, rvRNAs drive significantly higher levels of heterologous protein expression.
In our study, we present an RNA-based vaccine platform that merges rvRNA technology with a novel nanoparticle delivery vehicle called a Nanostructure Lipid Carrier (NLC). The current leading approach in mRNA delivery uses Lipid Nanoparticles (LNPs) – a combination of highly positively charged and neutral lipids that encapsulate RNA to shield from enzymatic degradation (Cullis & Hope 2017). Despite their popularity, LNPs face significant manufacturing challenges, with the final encapsulated product exhibiting a limited shelf-life (Ball et al. 2017). This may limit the use of LNP technology for large scale pandemic responses when millions of doses are needed quickly. The NLC formulation developed in this study is highly stable at room temperature. At the time of publication, we had demonstrated stability at 9 months and we have since demonstrated stability over 1 year. NLCs use the same industrial-scale processes employed in the manufacture of emulsion adjuvants at several million doses per batch. RNA binds to the NLC surface via electrostatic association with cationic lipids, therefore the formulation can be stockpiled and mixed with any target RNA molecule at the time of administration similar to what has been described before (Brito et al. 2014). We also describe compositions of NLCs that enhance the immunogenicity of the vaccine candidate in an adjuvant-like manner.
We combined the NLC formulation with rvRNA hoping that its self-amplifying properties would enable significant dose sparing. For proof-of-principle, we applied this approach towards a Zika vaccine. There is an immediate need for candidates that could prevent the spread of Zika virus and the catastrophic congenital syndromes it causes. As there are currently nucleic-acid strategies in the Zika vaccine pipeline, we benchmarked our approach against these. Immunogenicity studies in mice vaccinated with rvRNA delivered with our lead NLC formulation resulted in high titers of neutralizing antibodies, as measured by 80% plaque reduction neutralization test, with 100% seroconversion after a single intramuscular injection of only 10ng rvRNA. Following challenge with wild-type Zika virus, all NLC-formulated groups were protected from weight loss and death, while doses as low as 10 ng completely protected from detectable viremia. At even lower doses – 3 ng – we observed partial protection but significant reduction in viremia compared to mock vaccinated mice. In contrast, the leading Zika vaccine candidate, an LNP formulated, non-replicating modified mRNA, induces similar immunogenicity and efficacy following two 10g doses (Pardi et al. 2017). We hypothesize that this increase in potency using our approach may be due to the combination of the replicating viral RNA strategy and the adjuvant properties of the delivery formulation. Additional work will be needed to support this hypothesis.
Due to potential toxicity and reactogenicity associated with cationic lipids, lowering the dose is critical to safe clinical translation of RNA technologies. Our study shows that combining rvRNA with an effective delivery vehicle can result in more than a hundred-fold reduction in dose and potentially halves immunization frequency, lowering the total amount of formulation required. The study highlights the complementary roles of rvRNA and adjuvanted formulation as a strategy to reduce toxicity and suggests that this approach warrants further pre-clinical development.
References
Ball, R.L., Bajaj, P. & Whitehead, K.A., 2017. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. International journal of nanomedicine, 12, pp.305–315.
Brito, L.A. et al., 2014. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Molecular therapy : the journal of the American Society of Gene Therapy, 22(12), pp.2118–29.
Cullis, P.R. & Hope, M.J., 2017. Lipid Nanoparticle Systems for Enabling Gene Therapies. , 25(7), pp.1467–1475.
Pardi, N. et al., 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, 543(7644), pp.1–16.
Wolff, J. et al., 1990. Direct gene transfer into mouse muscle in vivo. Science, 247(4949), pp.1465–1468.
Xiong, C. et al., 1989. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science, 243(4895), p.1188 LP-1191.
Society News
 Presidential Symposium on Wednesday, May 1, 2019
Presidential Symposium on Wednesday, May 1, 2019
George M. Church, PhD—Harvard Medical School
George M. Church, PhD, is professor of genetics at Harvard Medical School, a founding member of the Wyss Institute, and director of PersonalGenomes.org, the world’s only open-access information on human genomic, environmental, and trait data. Church is known for pioneering the fields of personal genomics and synthetic biology. He developed the first methods for the first genome sequence and dramatic cost reductions since then (down from $3 billion to $600), contributing to nearly all “next generation sequencing” methods and companies. His team invented CRISPR for human stem cell genome editing and other synthetic biology technologies and applications—including new ways to create organs for transplantation, gene therapies for aging reversal, and gene drives to eliminate Lyme Disease and Malaria.
Church is director of IARPA & NIH BRAIN Projects and National Institutes of Health Center for Excellence in Genomic Science. He has coauthored 450 papers, 105 patents, and one book, “Regenesis.” His honors include the Franklin Bower Laureate for Achievement in Science, the Time 100, and election to the National Academies of Sciences and Engineering.
 George Stamatoyannopoulos Lecture on Tuesday, April 30, 2019
George Stamatoyannopoulos Lecture on Tuesday, April 30, 2019
Michel Sadelain, MD, PhD—Memorial Sloan Kettering Cancer Center
Michel Sadelain, MD, PhD, is the director of the Center for Cell Engineering and the incumbent of the Stephen and Barbara Friedman Chair at Memorial Sloan-Kettering Cancer Center. He is a member of the Immunology Program and the Departments of Medicine and Pediatrics.
Sadelain’s research focuses on human cell engineering and cell therapy to treat cancer and hereditary blood disorders. His laboratory has made several seminal contributions to the field of chimeric antigen receptors (CARs), from their conceptualization and optimization to their clinical translation for cancer immunotherapy. His group was the first to publish dramatic molecular remissions in patients with chemorefractory acute lymphoblastic leukemia following treatment with autologous CD19-targeted T cells.
Sadelain is the recipient of the Cancer Research Institute’s Coley Award for Distinguished Research in Tumor Immunology, the Sultan Bin Khalifa International Award for Innovative Medical Research on Thalassemia and NYPLA Inventor of the Year award. He previously served on the NIH Recombinant DNA Advisory Committee and as president of the American Society for Gene and Cell Therapy.
Free ASGCT and Cell Press Webinar October 3rd
Join Drs. David A. Williams, ASGCT member Adrian Thrasher, and ASGCT board member Alessandra Biffi for a Cell Press webinar on the evolution of hematopoietic stem cell gene therapy. Register today!
.png.aspx?lang=en-US) A new NIH proposal would remove Recombinant DNA Advisory Committee oversight from the gene therapy approval process. Upon review, ASGCT fully supports the change and will publish an editorial in an upcoming issue of Molecular Therapy...read more
A new NIH proposal would remove Recombinant DNA Advisory Committee oversight from the gene therapy approval process. Upon review, ASGCT fully supports the change and will publish an editorial in an upcoming issue of Molecular Therapy...read more
Public Policy
ASGCT Leaders and Members to Provide Recommendations to FDA
ASGCT’s first annual liaison meeting with the FDA’s Center for Biologics Evaluation and Research is occurring today. Liaison meetings provide an opportunity to discuss topics of mutual interest in the field. This year the topics that ASGCT members are addressing include testing methods and depth recommendation for off-target analysis of gene editing technologies, as well as three topics that relate to the gene therapy guidance documents the FDA recently issued: RCR and RCL testing of drug product; manufacturing considerations; and data from long-term follow-up for persistent vs. transient gene therapy product classes on critical safety parameters. Wilson Bryan, MD, the director of CBER’s Office of Tissues and Advanced Therapies, will present an update on the Regenerative Medicine and Advanced Therapies (RMAT) designation.
ASGCT will also be submitting comments on the six gene therapy guidance documents the FDA issued in July by the extended deadline of December 10. The ASGCT Clinical Trials & Regulatory Affairs Committee will consider feedback from all interested ASGCT members when compiling Society comments. To indicate interest in providing input, please contact ASGCT Senior Manager of Advocacy and Outreach, Betsy Foss-Campbell, on or before October 10 with your name, title, affiliation, and relevant topics of interest (hemophilia, retinal disorders, other rare diseases, and/or manufacturing and testing recommendations for gene therapy products).
NIH and FDA Supports NIH RAC Proposal
Last month the NIH proposed to amend the NIH Guidelines for Research Involving Recombinant and Synthetic DNA Nucleic Acid Molecules (NIH Guidelines). The proposed changes include eliminating Recombinant DNA Advisory Committee (RAC) review and reporting requirements to NIH for human gene therapy protocols.
ASGCT largely supports these proposed changes to increase the efficiency of regulatory oversight of gene therapy products. The RAC has served a valuable role, and will continue to do so, as a public forum for discussions of science, safety, and ethics. However, the current requirements for gene therapy protocol submission and subsequent reporting to both NIH and FDA are duplicative and are not required for other areas of clinical research. Safety and patient access are paramount to ASGCT, and the Society has full confidence in the FDA’s comprehensive oversight mechanisms to ensure both the safety and expeditious development of gene therapy.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet