Viewing Breast Cancer with a CRISPR Lens: The Potential of Gene-Editing Treatment
Eoghan J. Mulholland, Ph.D. - October 20, 2020
To recognize World CRISPR Day and International Breast Cancer Awareness Month, Dr. Mulholland explores how the Nobel prize-winning tool is helping to fight the disease.
As we moved into the month of October the days certainly became CRISPR…. on the 7th of October 2020 Professors Jennifer Doudna and Emmanuelle Charpentier made history when they became the first two women to be awarded the Nobel Prize for chemistry. Their work of course focuses on the revolutionary gene-editing technology: CRISPR-Cas9. October’s International Breast Cancer Awareness Month coincides with World CRISPR Day on October 20, and so let’s discuss how the Nobel prize winning tool is helping to tackle breast cancer.
What is CRISPR-Cas9 and how does it work?
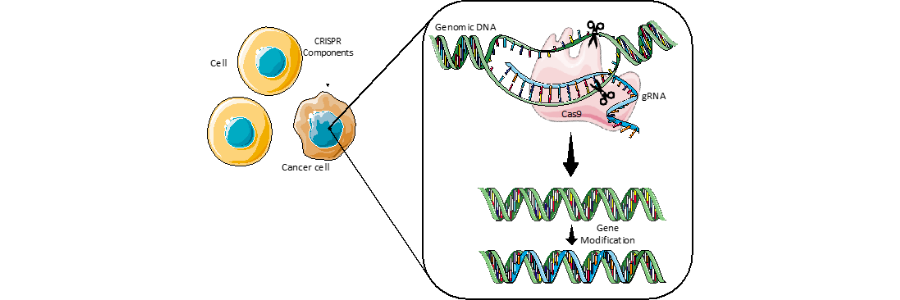
CRISPR stands for clustered regularly interspaced short palindromic repeats, and can be characterised as a prokaryote immune system, meaning it is a tool used by bacteria and archaea to counteract infection by viruses. Fundamentally, CRISPR evokes bacteria with the capabilities to recognise precise genetic sequences matching that of the invading virus and destroy it using specialised enzymes. Engineered CRISPR tools contain two components: a guide RNA (gRNA) and a CRISPR-associated endonuclease. The former is a short synthetic RNA that contains a scaffold, allowing it to bind with a the CRISPR-associated endonuclease, which is often referred to as ”molecular scissors” allowing for DNA to be cut at specific points. The gRNA contains a ~20 nucleotide spacer which allows for definition of the genomic target for modification. Therefore, the target of the system can be modified for purpose depending on the target sequence of the gRNA.
In short, the technology can be delivered into cells to modify genomic DNA of choice, which holds amazing potential in the treatment of genetic diseases such as cancer or cystic fibrosis.
Breast cancer
October is International Breast Cancer Awareness Month, and the disease is expected to effect approximately 1 in 8 women in the United States with invasive disease during their lifetime; in 2020, this included 276,480 new cases in addition to 48,530 new cases of non-invasive breast cancer. But it is not just women who can be affected by this disease, it is estimated that more than 2,600 men will be diagnosed with new cases of invasive breast cancer in 2020.
Genetically, breast cancer is a mine field. Some forms of breast cancer are familial, caused by inherited mutations in specific genes like BRCA1 or BRCA2. These genes produce proteins which are fundamental in fixing damaged DNA, thus if mutated, genetic stability would be compromised, resulting in rapid and uncontrolled cell division.
Many breast cancers are susceptible to your body's own hormones: estrogen and progesterone. Knowing the hormone status of the cancer allows for the clinical evaluation of treatment options most likely to work.
Breast Cancer hormone statuses includes: Estrogen receptor (ER) positive, Progesterone receptor (PR) positive, and Hormone receptor (HR) negative, the last of which occurs when the cancer doesn't have hormone receptors, so it won't be affected by endocrine treatments aimed at blocking hormones in the body. Additionally, it is typical to characterise breast tumours in terms of HER2 status, a gene which, when abundant, leads to excessive growth-promoting HER2 protein. The condition is thankfully treatable with drugs. Unfortunately, a separate class of breast cancer termed Triple Negative breast cancer (TNBC) is hormone receptor negative in addition to HER2 negative, making it more challenging to treat.
How CRISPR can help in the fight against breast cancer
With the above insight in mind, CRISPR technology could be utilised in the battle against breast cancer given its genetic dysregulation. Indeed, in a project conducted at the Boston Children’s Hospital, researchers led CRISPR delivery experiments to human tumour cells implanted in mice. This particular experiment was a model of triple-negative breast cancer and results showed that the CRISPR system had the capabilities to target the tumour tissue and knock-out the Lipocalin-2 oncogene. Furthermore, the investigators found no adverse toxicity issues in normal tissue. These findings were published here.
I had the pleasure of speaking with Dr. Florijn Dekkers, a postdoctoral researcher at the Princess Máxima Center for Paediatric Oncology, the Netherlands. Dr Dekkers is currently working on improving cellular immunotherapy targeted to solid tumours using advanced 3D live cell imaging and human cancer organoid technologies. She had the following to say on how CRISPR is influencing her field of research:
“With this unique technology researchers are now able to specifically edit genes and assess their role in breast cancer on onset, development as well as metastases formation in order to identify targets driving breast cancer development and improve therapies,” Dekkers said. “In the immune-oncology field, the technology [CRISPR-Cas9] holds promise for ex vivo engineering of immune cells and injecting these cells into patients for specific targeting of breast cancer.”
Taken together, CRISPR-Cas9 is generating great shifts in the right direction for the study of Breast Cancer and beyond. Not only is it a promising tool for treating disease directly, but also as a means of modifying patient cells ex vivo. Evidently, the future of medicine through a CRISPR lens is clear.
Dr. Mulholland is a postdoctoral research scientist in cancer genetics and Junior Research Fellow (Somerville College) at the University of Oxford. Dr Mulholland is also a member of the ASGCT Communications Committee
Reflections on CRISPR-Cas9
ASGCT Communications Committee members asked their colleagues to share thoughts on the recent Nobel Prize announcement and how they feel the award will impact development of therapeutics and public perception of the technology. Here are the responses they collected:
"In due recognition for their significant contributions to the field of genome editing, Drs. Emmanuelle Charpentier and Jennifer A. Doudna were awarded the 2020 Nobel Prize in Chemistry. The retooling of the CRISPR/Cas9 system to recognize and edit DNA at specified sites has revolutionized the way in which we now study basic biology, increased the speed at which we are able to perform genetic screens and generate new disease models, and inspired novel types of therapeutics, including many ways in which T cell therapies will be generated in the future. In the nine years since the discovery of this editing system, CRISPR has become an important asset for the scientific industry, with an impact comparable to introduction of methods like polymerase-chain reaction (PCR) and Sanger sequencing, two notable methods also recognized by the Nobel Prize in Chemistry in prior years. The fact that these Nobel laureates, the 55th and 56th women to be awarded Nobel prizes, are dynamic scientists and inspirational entrepreneurs who will inspire generations of future scientists is the icing on this year’s celebratory Nobel cake."
Avery D. Posey, Jr., Ph.D.
Assistant Professor
Systems Pharmacology and Translational Therapeutics
University of Pennsylvania Perelman School of Medicine
“My colleagues and I are delighted that the Nobel committee has recognized the work of Emmanuelle Charpentier and Jennifer Doudna in discovering CRISPR/Cas9’s potential as an accessible and versatile tool with unparalleled potential for impact across a wide range of fields. The cell and gene therapy community is only beginning to fully leverage this platform to cure disease and it is clear that it will serve as the basis for more precise gene engineering for decades to come. The fact that this discovery is attributed to two female scientists is just icing on an already very sweet cake…such an inspiration to us all, especially young female scientists who need more role models at the highest echelons of science.”
Crystal Mackall, M.D.
Ernest and Amelia Gallo Family Professor of Pediatrics and Internal Medicine
Pediatrics - Hematology & Oncology
Stanford University
"The groundbreaking power and versatility of the precise genome-editing CRISPR-Cas9 technology Doudna and Charpentier developed has opened up novel and wide-ranging potential for the treatment of a multitude of intractable diseases. The fact that Doudna and Charpentier this week became the first women in history to win a Nobel Prize in the sciences together further emphasizes how they are not only pioneers in the gene therapy field but are also trailblazers for the next generation of female scientists!"
Catherine Bollard, M.D., M.B.Ch.B.
Director, Center for Cancer and Immunology
Director, Program for Cell Enhancement and Technologies for Immunotherapy
Children's National Hospital
Related Articles