Start Your Engines: The Hemophilia Drug Race is On
Eoghan J. Mulholland, Ph.D. - April 17, 2020
World Hemophilia Day 2020, which falls on April 17, aims to raise awareness of this complex disorder, and gene therapy is at the forefront of providing an exciting treatment option.
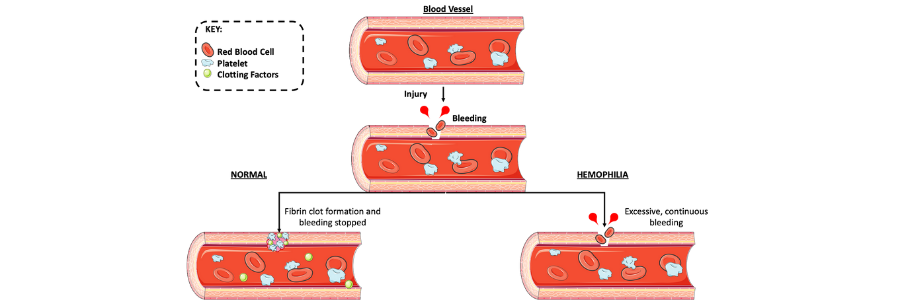
Hemophilia is a rare X-linked genetic disorder characterized by blood having a reduced ability to clot due to a lack of sufficient clotting factors. The disorder arises from defects in the F8 or F9 genes which leads to either hemophilia A (HA) or hemophilia B (HB) respectively. Those with HA lack the clotting factor VIII, whereas HB patients lack IX.
Those with hemophilia may bleed for prolonged periods of time after injury. Furthermore, patients with severe disease are prone to internal bleeding, which typically occurs in the knees, elbows, and ankles. These internal bleeds can lead to organ and tissue damage that can be fatal if it’s not treated. April 17 marks World Hemophilia Day 2020, which aims to raise awareness of this complex disorder. Current treatments have had a revolutionary effect on the management of this disease, but have many associated caveats. The race is on for novel, highly efficient treatments. In this regard, gene therapy is at the forefront of providing an exciting treatment option to combat this devastating disease.
Symptoms of hemophilia
The symptoms of patients with hemophilia vary widely depending on the level of clotting factor deficiency. For example, mild hemophilia may result in excessive bleeding after surgery or trauma, whereas severe hemophilia patients may experience spontaneous bleeding.
Signs and symptoms of severe disease include the following, according to the Mayo Clinic:
- Unexplained and excessive bleeding from cuts or injuries, or after surgery or dental work
- Many large or deep bruises
- Unusual bleeding after vaccinations
- Pain, swelling, or tightness in your joints
- Blood in your urine or stool
- Nosebleeds without a known cause
- In infants, unexplained irritability
The danger of brain hemorrhage
A slight bump to the head can cause hemorrhage (bleeding) within the brain for many suffering with severe hemophilia. Occurrences of this specific trauma are rare, but need to be treated very seriously. Signs and symptoms of brain hemorrhage include:
- Painful, prolonged headache
- Repeated vomiting
- Sleepiness or lethargy
- Double vision
- Sudden weakness or clumsiness
- Convulsions or seizures
Current treatments for hemophilia
Replacement therapy is the main treatment for hemophilia. Solutions of clotting factors are delivered to patients intravenously by injection or drip. These clotting factors are derived from human blood or synthesised via recombination. Clotting factors can be easily administered at home and are easily stored. Replacement therapy may need to be taken prophylactically on a regular basis to prevent bleeding, or it may only be required at the point when bleeding occurs. As effective at this therapy is, there are many complications that can arise from replacement of clotting factors, including:
- Development of an immune response to the clotting factors (also known as inhibitor induction)
- Virial infections from human clotting factors (low risk due to stringent screening of donated blood)
- Damage to joints, muscles, or other parts of the body if there are delays in treatment
Alternative treatments
- Desmopressin is a synthetic hormone used to treat mild HA. Desmopressin works by stimulating the release of stored clotting factor VIII as well as Von Willebrand factor, which carries and binds clotting factor VIII, allowing it to remain for longer periods in the blood stream. This drug can be taken by injection or nasal spray, but since the effects are short lived, they are only applicable to certain situations like before playing sports as a preventative measure.
- Antifibrinolytic drugs are used to help keep blood clots from degrading and can be used in conjunction with replacement therapy. These drugs are often used to tend to nose bleeds or mild intestinal bleeding.
- Treating specific bleeding sites can include anything from pain medication to physical therapy to help alleviate pain and swelling at the affected site.
Gene therapy for hemophilia
Hemophilia is regarded by many to be an ideal candidate for gene therapy due to the monogenic nature of the disease. Furthermore, it has been observed that even minor increases in clotting factor levels can have a significant impact on patient's quality of life.
There are currently 39 clinical trials listed under gene therapy and hemophillia on www.clinicaltrials.gov, which is an excellent indicator of a promising field.
BioMarin Pharmaceutical currently has an exciting gene therapy option for hemophilia with the FDA for ruling, with the outcome to hopefully be decided this coming summer. The drug in question is called Valoctocogene roxaparvovec and, if approved, it would be the first-ever gene therapy for HA. BioMarin is exploring prices for this therapy at between $2 million and $3 million, which could make it the most high-priced drug on the planet. This is a title currently held by Zolgensma, another gene therapy that treats spinal muscular atrophy and has a list price of $2.1M million.
I spoke with Graça Almeida-Porada, M.D., Ph.D., who is a professor and the director of fetal research and therapy at the Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, North Carolina. She had the following insight into the use of gene therapy for hemophilia and the much anticipated Valoctocogene roxaparvovec:
“We are now in the year 2020, and a better therapy for Hemophilia A patients is long overdue; one that is safe, affordable, provides a high quality of life, and can promise long-lasting, ideally lifelong, correction. Gene therapy is one of the key options we have right now that could fulfill this promise, providing lifelong cure following a single treatment, and perhaps circumventing the risk of inhibitor induction. The current cost for this treatment is predicted to be $2-3 million, which may, at first blush, appear quite expensive. However, if one considers that the cost of the current standard of care, prophylactic factor replacement, is $300,000-$500,000 per year, the price for a permanent cure suddenly seems quite reasonable.”
Dr. Almeida-Porada continued: “If BioMarin’s ongoing trial proves that their gene therapy-based treatment is safe, effective, and long-lasting, the next step would be to shift the time for intervention to shortly after, or even prior to, birth. Because the cost of therapy is driven largely by the amount of vector that needs to be made to treat a fully-grown adult patient, treating shortly after birth would drastically reduce the amount of vector required to achieve the same dose/kg, and correspondingly lower the cost of the treatment. Better still would be to treat prior to birth, which would exponentially lower the amount of vector, further reducing the cost. More importantly, a shift to treating early in life would make it possible to correct hemophilia before the disease had a chance to produce any untoward effects on the patient, avoiding all of the suffering and hardships that this chronic disease brings, and enabling these deserving patients to live their lives to the fullest.”
The results from the phase III clinical trial for Valoctocogene roxaparvovec were extremely promising. Results showed that with treatment there was an 85% reduction in annual bleed rates when also receiving standard prophylaxis.
With the concept of a drug race in mind, it’s worth noting that other companies are close behind BioMarin in developing alternative gene therapy options for hemophilia. Spark Therapeutics (Roche) has a therapy called SPK-8011 in phase III trials for HA. Furthermore, they are also developing SPK-9011 for HB, a project in partnership with Pfizer Inc.
Evidently, gene therapy is on the racetrack to becoming the gold standard of hemophilia treatment. With current treatments requiring routine administration and having many potential drawbacks (as well as hefty annual costs), gene therapy could help solve all these issues in the short and long term.
Dr. Mulholland is a postdoctoral research scientist in cancer genetics at the University of Oxford and a member of the ASGCT Communications Committee.
Related Articles