The Vector
Volume 8, Issue 2: February 2019
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
President's Message
Breaking Through
Society News
Public Policy
Industry News
President's Message
Patient Education on Gene Therapy Disease Treatments Comes to ASGCT.org

The first of our disease-specific patient education materials, covering spinal muscular atrophy and X-linked myotubular myopathy, are now available on ASGCT.org and three more pages are yet to come! By the end of the month, the Society will have patient education videos covering leukodystrophy, inherited retinal disorders, and blood disorders.
The Patient Education portal is a new initiative for 2019 and was designed by ASGCT committee volunteers in coordination with patient advocacy groups. The program is designed to educate and inform patients, families, and the public on the status and promise of gene and cell therapies.
The videos, infographics, and treatment progress reports have been commended and shared by patient advocacy groups, trade media, and the public. I encourage all of our members to spend some time with these materials and share them with your friends and family—they’re a great way to explain our field to people who may not have a high level of exposure to science, especially some of the more complicated areas in which many of us spend our time.
And as we spend that time, I’m looking forward to seeing many of you at the 23rd Annual Meeting. While just a couple of rooms remain in the ASGCT hotel block at the Washington Hilton host hotel, early registration will remain open through April 6 and includes a 15 percent discount on all rates. You can also add on a pre-meeting workshop to your registration to earn an additional $25 discount on your workshop registration. These four workshops, CAR T and Related Immune Effector Cell Therapies, Gene Editing, Pre-Approval Commercialization, and Post-Approval Commercialization, are all valuable opportunities to dig deeper into our booming field and to meet likeminded colleagues from around the world. I hope you’ll consider taking advantage of these fantastic educational and professional opportunities.

Breaking Through
Novel Chimeric Gene Therapy Vectors Based on Adeno-Associated Virus and Four Different Mammalian Bocaviruses
Julia Fakhiri, Marc A. Schneider, Jens Puschhof, Megan Stanifer, Verena Schildgen, Stefan Holderbach, Yannik Voss, Jihad El Andari, Oliver Schildgen, Steeve Boulant, Michael Meister, Hans Clevers, Ziying Yan, Jianming Qiu, Dirk Grimm
Summary by Dr. Dirk Grimm and Julia Fakhiri
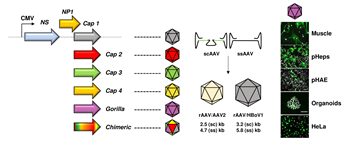 Vectors derived from the Parvoviridae virus family have been studied for decades as tools for gene transfer and have demonstrated great promise for a variety of applications in cancer, immuno- and gene therapy. As compared to other DNA viruses, a major reason for their success is the unique simplicity of the parvoviral genome—typically a single-stranded DNA with a length of 4.7 to 6 kilobases (kb)— which facilitates genetic manipulation and vector tailoring. On the one hand, this has culminated in the design of "gutless" vectors that are fully devoid of viral genes, a concept that particularly characterizes the current generation of recombinant adeno-associated virus (AAV) based-vectors. On the other hand, it has enabled the implementation and application of different technologies that aim at increasing the fitness and specificity of parvoviral vectors via modification of the viral capsid (gene).
Vectors derived from the Parvoviridae virus family have been studied for decades as tools for gene transfer and have demonstrated great promise for a variety of applications in cancer, immuno- and gene therapy. As compared to other DNA viruses, a major reason for their success is the unique simplicity of the parvoviral genome—typically a single-stranded DNA with a length of 4.7 to 6 kilobases (kb)— which facilitates genetic manipulation and vector tailoring. On the one hand, this has culminated in the design of "gutless" vectors that are fully devoid of viral genes, a concept that particularly characterizes the current generation of recombinant adeno-associated virus (AAV) based-vectors. On the other hand, it has enabled the implementation and application of different technologies that aim at increasing the fitness and specificity of parvoviral vectors via modification of the viral capsid (gene).
To date, one can distinguish two diverse approaches to enhance parvoviral vector features through the capsid. The first, by direct genetic modulation of the latter by e.g., introducing small peptides (peptide display) or creating chimeric capsids composed of two or more virus serotypes (DNA family shuffling). Alternatively, the repertoire of parvoviral vectors can be expanded through pseudotyping of the viral genome with a natural capsid that is derived from a different parvoviral variant. This can result in (i) inter-genera combinations where a recombinant AAV2 genome is packaged into a capsid from another AAV serotype, or in (ii) cross-genera constellations, in which viral genome and capsid originate from two distinct parvoviral genera. These so-called hybrid vectors ideally combine the best features of both worlds, in particular the low genotoxicity of AAV2 genomes and desirable properties of the selected capsids. Examples comprise the encapsidation of AAV genomes into capsids of parvovirus B19 or human bocavirus 1 (HBoV1), yielding AAV/B19 or AAV/HBoV1 vectors that exhibit unique assets (in this nomenclature, the virus that is mentioned first represents the packaged genome and the second one the capsid). Moreover, we note one report in which a parvovirus H1 genome was cross-packaged into AAV2 capsids, in order to harness the tumor-specific expression profile of H1 from a viral shell with a broader tropism.
While encouraging, two limitations have hampered the wider use of hybrid parvoviral vectors thus far, i.e., (i) the low viral titers, which mostly restricted their application to cell culture experiments, and (ii) the technical challenge of pairing two distinct viruses, which depends on a delicate balance of compatibility and plasticity. Moreover, success requires a profound understanding of virus biology to ensure proper genome packaging and vector transduction. Notably, pioneering work from the labs of Ziying Yan, Jianming Qiu and John Engelhardt has suggested solutions to these hurdles, by demonstrating the feasibility to not only package large single-stranded (ss)AAV2 genomes into HBoV1 capsids, but to also fine-tune the production protocol and to thereby obtain titers that are up to 10.000-fold higher than those reported originally, and that now reach the levels of genuine rAAV vectors. In addition, their work highlighted the inherent tropism of HBoV1 for the airways and its general preference for transduction from the apical surface. This makes this vector attractive for gene therapy applications in the lung, as verified in an in vivo study in ferrets that proved the safety and efficiency of this hybrid viral system. Finally, akin to AAV, there are numerous naturally occurring variants of BoV that could be used to package and deliver AAV genomes, promising options to target multiple other cells and tissues beyond the lung.
In a new study published in Molecular Therapy - Methods & Clinical Development, Fakhiri and co-workers from the Grimm lab have capitalized on these promises and validated a number of additional benefits of AAV/BoV vectors. Firstly, we explored their packaging limit and found that oversized AAV genomes of up to 5.8 (ssAAV) or 3.2 kb (self-complementary [sc]AAV) can be packaged into the HBoV1 capsid, whereas AAV capsids package only up to 4.7 (ssAAV) or 2.5 kb (scAAV). The gain mediated by the BoV capsid allows to encapsidate larger transgenes, as shown before for the cystic fibrosis transmembrane regulator (CFTR) or, here, for the whole CRISPR/Cas9 machinery. Next, we further extended the AAV/HBoV1 system, by exploiting four additional primate BoVs—three from humans (HBoV2, 3 and 4) and one from Gorilla (GBoV)—that have not been studied as vectors before. Akin to HBoV1, we were able to produce hybrid AAV/BoV vectors of all studied serotypes, expressing two different reporter genes. To characterize the as-of-yet unknown cell specificity of the newly cloned BoV variants, we conducted various screens and found that especially GBoV has a much wider tropism in vitro than previously anticipated. In particular, a range of primary cells were efficiently transduced by the different BoVs, including human hepatocytes, T-cells and skeletal muscle cells. In addition, we obtained the first evidence that pseudotyped AAV/BoV vectors not only differ in cell specificity, but also in reactivity to pooled human antibodies (IVIg). These initial data are very encouraging from a clinical perspective as they imply the possibility of vector re-dosing in patients, identical to the strategy used in the AAV field.
Finally, we also explored the possibility of using directed molecular capsid evolution, a method commonly applied for the diversification of AAV capsids with the aim to select for candidates with new and desired properties. In our study, we demonstrate that DNA family shuffling can be successfully applied to all five BoV capsids and results in a highly diverse and packaging-competent BoV library. Subjecting this library to different selection pressures should now allow for the identification of designer BoV vectors with desirable features not occurring in nature, such as new tropism, greater efficiency and/or lower immunoreactivity.
Collectively, the data and strategies presented in this new work offer a first insight into the biology of the different BoV isolates and their potential application as gene transfer vehicles. Together with the profound experience with AAV vectors gathered over five decades, the rapidly accumulating knowledge of BoV biology will foster the rational improvement of the hybrid AAV/BoV system. Thus, there is every reason to believe that remaining hurdles will soon be overcome and to be excited over this emerging gene delivery system.
Society News
ASGCT on SciShow
ASGCT has partnered with SciShow to produce three episodes of the hit educational YouTube series focused on gene therapy. The first of these episodes, Changing DNA in a Cell With No DNA: Gene Therapy for Blood Disorders, was released late last month. Videos on fetal gene editing and developing gene therapy to treat addiction will release in February and March, respectively.
2.5 Million Euro Funding Opportunity Accepting Applications
The 2020 Else Kröner Fresenius Award for Medical Research is accepting applications in the fields of gene editing and gene therapy through April 4. The biennial research will distribute 500,000 Euro directly to the recipient with an additional 2 million Euro dedicated to the awardee’s research efforts. Candidates “must be researchers who have made significant scientific contributions to the field of genome editing and gene therapy and are expected to yield groundbreaking discoveries in the near future.”
Learn more about the Else Kröner Fresenius Award for Medical Research
Public Policy
Senate Bill Would Enable Value-Based Purchasing Arrangements
Late last month, Senators Bill Cassidy (R-LA) and Mark Warner (D-VA) unveiled draft legislation that would allow innovative payment methods for covered outpatient drugs, including gene therapy This draft bill, titled the Patient Affordability, Value and Efficiency (PAVE) Act, would remove regulatory and legal barriers to value-based purchasing arrangements (safe harbors from the Anti-Kickback Statute and Stark Law, and exemption from Medicaid best price determinations).
ASGCT supports the removal of barriers to payment arrangements that tie some, or all, of the reimbursement for a gene therapy to meeting predetermined outcomes. The Society recommends addressing that such arrangements could also include an installment payment provision for gene therapies, which would address the unique, potentially one-time administration of some gene therapies.
ASGCT’s support of components of the proposed bill does not imply endorsement of specific gene therapy pricing decisions. ASGCT will submit written comments next week, as requested by the Senators’ offices.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet