Welcome Wan Du, MD, PhD: Vector Junior Editor
Wan Du, MD, PhD - October 13, 2023
Wan Du, MD, PhD, is a postdoctoral research fellow at Mass Eye & Ear/Harvard Medical School. Read her first Breaking Through article summary on an mRNA LNP vaccine against Lyme disease.
Development of an mRNA-lipid nanoparticle vaccine against Lyme disease
Pine M, Arora G, Hart TM, Bettini E, Gaudette BT, Muramatsu H, Tombácz I, Kambayashi T, Tam YK, Brisson D, Allman D, Locci M, Weissman D, Fikrig E, Pardi N
DOI: https://doi.org/10.1016/j.ymthe.2023.07.022
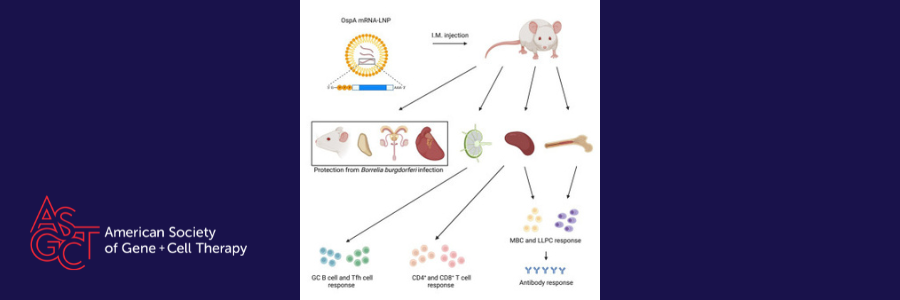
Lyme disease is the most prevalent vector-borne disease, primarily caused by different species and strains of the Borrelia burgdorferi sensu lato bacteria transmitted to humans via tick bites. The symptoms of Lyme disease include fever, fatigue, headache, and a skin rash. Without proper treatment, the infection can spread to the joints, heart, and nervous system. While most Lyme disease cases respond well to a few weeks of antibiotics, a subset of individuals may experience post-treatment Lyme disease syndrome (PTLDS), characterized by persistent symptoms like severe joint pain and neurocognitive problems. What's even more concerning is that being infected with one strain doesn't provide immunity against different strains. Therefore, the development of a comprehensive preventive vaccine is essential to stop both new and recurrent cases of Lyme disease. B. burgdorferi produces a variety of proteins that could potentially serve as vaccination targets. One of these proteins is outer surface protein A (OspA), which is a surface lipoprotein abundantly present and responsible for attaching the bacterium to the tick midgut. It gets rapidly reduced during the feeding process, highlighting the importance of early targeting. In the United States, there are numerous distinct strains of B. burgdorferi that can infect humans, making OspA an attractive vaccine target due to its widespread conservation among these strains.
Back in 1998, LYMErix™, an alum-adjuvanted recombinant OspA (rOspA) protein vaccine, was released, demonstrating a 75% reduction in Lyme disease cases within a year. However, the vaccine was withdrawn from the market in 2002. Since then, there has been no Lyme disease vaccine approved by the FDA, despite a continuing increase in cases. In a recent study in Molecular Therapy, Matthew Pine et al. propose using an innovative strategy, mRNA-based lipid nanoparticle (mRNA-LNP) vaccine platform to develop a Lyme disease vaccine. The success of mRNA-LNP has extended to humans, as it prompts potent antibody and cellular immune reactions against SARS-CoV-2 virus.
The authors have designed and produced an mRNA-LNP vaccine encoding OspA and then assessed its immune response and protective effectiveness in comparison to an OspA protein subunit vaccine with alum adjuvant. The results indicated that mice received a single OspA mRNA-LNP dose exhibited stronger polyfunctional CD8+ and CD4+ T-cell responses compared to those vaccinated with the OspA protein. While the primary defense against B. burgdorferi infection in the host is through antibody responses, the presence of anti-OspA CD8+ T cells could offer additional immune reinforcement, particularly since some patients have spirochetes expressing OspA during late-stage infection. One specific subgroup of CD4+ T cells known as Tfh cells, as well as antigen-specific GCB cells were significantly greater increase in a single vaccination with OspA mRNA-LNP than with rOspA + alum. The enhanced magnitude of germinal center responses was associated with higher levels of their terminal outputs, including antigen-specific memory B cells (MBCs) and long-lived plasma cells (LLPCs). These cells secreted IgG1, IgG2a, and IgG2b antibodies following a single OspA mRNA-LNP immunization. Furthermore, with these potent immune responses, a single administration of OspA mRNA-LNP proved to be more successfully in protecting mice from B. burgdorferi infection compared to the recombinant protein counterpart.
Through further preclinical and clinical advancements, OspA mRNA-LNP may prove to be a feasible preventive strategy for reducing the prevalence of Lyme disease. Their findings also highlight the potential for developing highly effective mRNA vaccines against non-viral pathogens.
Welcome, Wan, to the Vector editorial team!
Read The Vector