ASGCT Congressional Briefing Highlights Transformative Potential of Gene Editing Technologies
Paula Cannon, PhD - June 10, 2025
Gene editing represents a paradigm shift in medicine, offering the possibility to address the root genetic causes of disease rather than just treating symptoms.
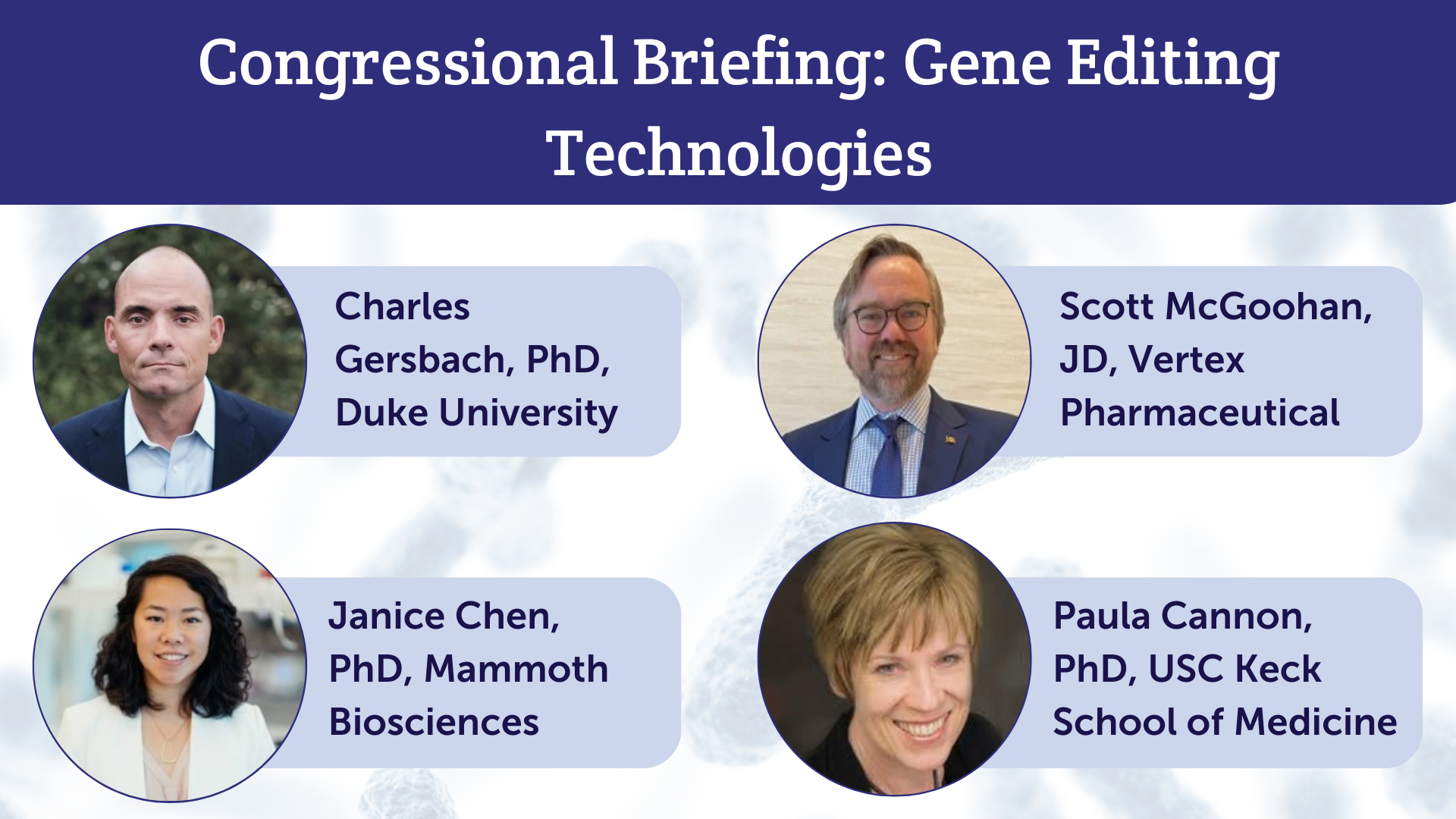
The American Society of Gene and Cell Therapy (ASGCT) hosted a congressional briefing April 9 on gene editing technologies, bringing together experts from academia and industry to educate congressional staff on this rapidly evolving field. The virtual event was designed to help policymakers understand what gene editing is, its role in the biomedical ecosystem, and the tremendous opportunities it presents for patients with genetic diseases. You can review the briefing slide deck here.
Gene editing represents a paradigm shift in medicine, offering the possibility to address the root genetic causes of disease rather than just treating symptoms. This is particularly significant for rare diseases, which affect approximately 30 million Americans and 350 million people worldwide. With approximately 80% of rare diseases having genetic origins, gene editing technologies hold tremendous promise for conditions that currently have few or no treatment options.
.aspx?width=500&height=281)
Dr. Charlie Gersbach (Duke University) opened the briefing with a comprehensive introduction to gene editing, tracing its evolution from traditional gene therapy approaches and explaining how these technologies can precisely modify DNA to address genetic diseases at their source. Building on this foundation, Dr. Janice Chen (Mammoth Biosciences) presented the rapidly expanding gene editing pipeline. She highlighted the first FDA-approved gene editing product, an ex vivo therapy for sickle cell disease, and explained the field’s evolution toward a broader range of diseases with in vivo treatment potential.
Transitioning from scientific innovation to practical implementation, Scott McGoohan (Vertex Pharmaceuticals) addressed the healthcare system challenges these revolutionary therapies present. His presentation outlined innovative coverage and reimbursement models that facilitate patient access to potentially curative treatments, highlighting how CGTs disrupt traditional healthcare payment structures. Finally, with my presentation we brought the patient experience to the forefront. I reminded congressional staff in attendance that gene editing isn’t theoretical - the first patients to benefit from gene editing are already here. I also highlighted the complex considerations—from treatment risks to long-term monitoring—that patients weigh when contemplating gene editing therapies.
Continued dialogue between scientists, patients, industry representatives, and policymakers is essential to ensure that scientific advancements in gene editing are supported by thoughtful policy development. ASGCT thanks all presenters for their contributions, along with the dedicated congressional staff for their participation and engagement on this critical topic.
Dr. Cannon is ASGCT's Immediate Past President and Distinguished Professor at USC Keck School of Medicine.