FDA Approves Patisiran, First-Ever RNA Interference Therapeutic Approved for Clinical Use
ASGCT Oligonucleotide and RNAi Therapeutics Committee - August 16, 2018
Infusions of Onpattro (Alnylam Pharmaceuticals) is the first therapeutic to treat peripheral nerve disease (polyneuropathy) caused by hereditary transthyretin-mediated amyloidosis (hATTR) approved by the U.S. Food and Drug Administration, in addition to the first targeted RNA-based gene therapy.
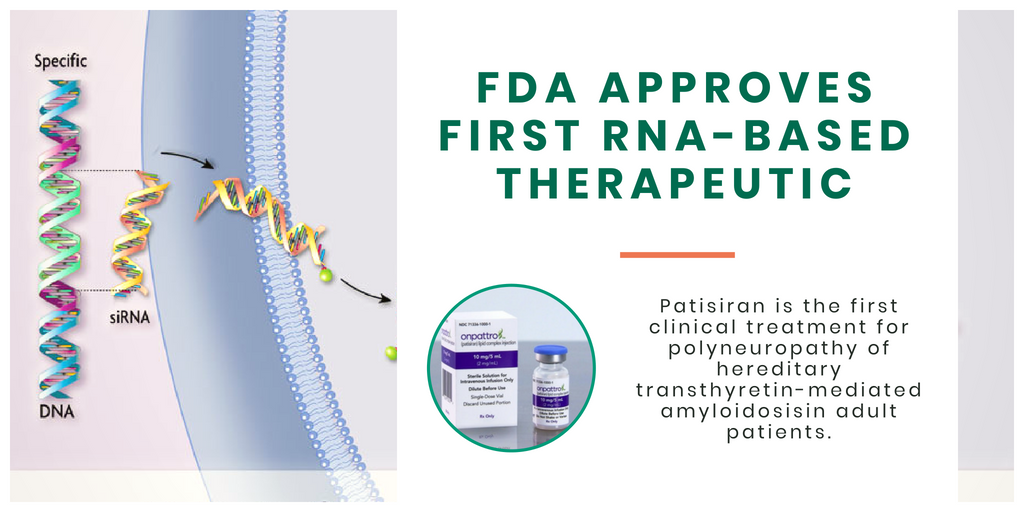
The U.S. Food and Drug Administration has approved infusions of Patisiran (Onpattro, Alnylam Pharmaceuticals) to treat peripheral nerve disease (polyneuropathy) caused by hereditary transthyretin-mediated amyloidosis (hATTR). It is the first-ever approval of a new class of drugs, small interfering ribonucleic acid (siRNA), used to treat rare and genetic diseases.
Polyneuropathy is a rare, debilitating, and often fatal genetic disease affecting approximately 50,000 people worldwide and it is characterized by the buildup of abnormal amyloid protein in peripheral nerves, the heart and other organs. Patisiran was previously granted numerous highlight designations by the FDA including Fast Track, Priority Review, Breakthrough Therapy, and Orphan Drug designations.
“This approval is part of a broader wave of advances that allow us to treat disease by actually targeting the root cause, enabling us to arrest or reverse a condition, rather than only being able to slow its progression or treat its symptoms,” FDA Commissioner Scott Gottlieb, M.D. said in a statement. “In this case, the effects of the disease cause a degeneration of the nerves, which can manifest in pain, weakness and loss of mobility.”
A Phase III study of Patisiran lasting 18 months revealed improvements in quality of live, daily activities, ambulation, and nutrition against a placebo in a global, randomized patient population.
Patisiran and other RNA interference (RNAi) therapies work by silencing specific genes that are the root cause of specific diseases. RNAi treatments are engineered to harness a natural cellular process and represent a novel form of disease treatment; Patisiran, part of a subset of those treatments called small interfering RNA (siRNA), prevents messenger RNA (mRNA) from creating the disease-causing proteins that build up in patients with polyneuropathy.
As a larger whole, RNAi therapies are an exciting platform technology for developing additional gene-silencing drugs to treat other genetic diseases.
“Today’s approval is significant in so many respects. It means the hATTR amyloidosis community of patients, families, caregivers and healthcare professionals in the United States now has a treatment option that offers renewed hope,” Isabelle Lousada, founder and CEO of the Amyloidosis Research Consortium said in a statement. “With an FDA-approved treatment now available, I am more optimistic than ever that we can increase awareness of this rare disease and encourage more people to get tested and receive the proper diagnosis.”
The American Society of Gene & Cell Therapy sincerely congratulates and thanks the researchers, developers, patients, patient advocates, and all involved with the creation and approval of Patisiran for their hard work and perseverance in advancing an important and revolutionary form of gene therapy.