Use of Genetic Material
As we describe the different approaches of gene and cell therapy we often say “genetic material” is used or delivered to cells. Genetic material is a broad term and there are different types that may be used.
Genetic Material most commonly refers to DNA or RNA. These are strings of molecules with the information to instruct cells to produce proteins. Proteins play an important role in how our body functions.
 DNA stores the genetic information so the cell can continue building proteins. It is permanent and stored in the nucleus in a form called chromosomes. DNA is made up of two strands and is much larger than RNA. Genes are specific sections of DNA that encode sequences (I.e. a set of instructions) for making proteins. Other sections of DNA can control when, where, and how much of the protein is made.
DNA stores the genetic information so the cell can continue building proteins. It is permanent and stored in the nucleus in a form called chromosomes. DNA is made up of two strands and is much larger than RNA. Genes are specific sections of DNA that encode sequences (I.e. a set of instructions) for making proteins. Other sections of DNA can control when, where, and how much of the protein is made.
RNA is a copy of the genetic information contained within DNA. While DNA is the permanent storage format of genetic material, RNA molecules help turn those instructions into proteins. After RNA is made within the nucleus of a cell, most RNA moves to the cytoplasm, which is the fluid space between the nucleus and cell membrane. There are many different types of RNA. Some RNA carries the instructions for making proteins, while other types of RNA can prevent proteins from being made. RNA molecules are only active for a limited period of time in the cell. Keep reading to learn more about the types of RNA used in RNA Therapies.

Breaking Down the Approaches
Gene Therapy is the use of genetic material to treat or prevent disease. Learn more about Gene therapy Basics or Vectors 101. Three common effects of gene therapies in cells are:
 Gene addition adds in a working gene that has the instructions for the cell to make more of the specific protein needed. Vectors, which are often viruses, are used to deliver the working gene to the cell’s nucleus, where the DNA is stored. This gene will now live in the nucleus which gives a greater chance of being permanent and is only given one time. Sometimes the therapy is designed for the new gene to insert itself into the main DNA storage while other times it will stay next to the main DNA storage, like an extra set of instructions.
Gene addition adds in a working gene that has the instructions for the cell to make more of the specific protein needed. Vectors, which are often viruses, are used to deliver the working gene to the cell’s nucleus, where the DNA is stored. This gene will now live in the nucleus which gives a greater chance of being permanent and is only given one time. Sometimes the therapy is designed for the new gene to insert itself into the main DNA storage while other times it will stay next to the main DNA storage, like an extra set of instructions.- Gene silencing is where the delivered genetic material prevents or inhibits the activity of a gene that is already present in a cell. Gene silencing often decreases the amount of a specific protein being made.
- Gene Editing corrects pieces of DNA by changing or deleting the information within the affected individual’s gene. Genetic material is sent to directly edit or change pieces of DNA already located within a cell to correct the protein being made by that DNA. Gene editing uses technology that is highly precise to make these types of changes. Learn more about Gene Editing.
Please note, gene therapy is a rapidly evolving field that changes in response to new scientific discoveries and from what is learned about treatments being tested in the clinic and laboratory. Below we highlight different types of gene therapies and strategies that are currently being tested and may be approved for use in humans. As DNA and RNA work together, there may be overlap in the way each is applied.
DNA therapy is the use of DNA that codes for the production of a specific RNA or protein to treat a disorder. To have a therapeutic effect, the DNA must be delivered to the nucleus of a cell, where it can then be used by the cell to affect protein expression. This gene will now live in the nucleus which gives a greater chance of being permanent and is typically only given one time.
- DNA plasmids are large, double-stranded circular DNA molecules that code for a therapeutic protein. DNA plasmids can be used for gene addition, vaccination, and cell therapy. A challenge for DNA plasmids is getting them into the cell and then into the nucleus for therapeutic benefit. Clinical trials are ongoing to test the safety and benefit of DNA plasmid therapeutics.
- Viral vectors are modified viruses used as vehicles to deliver therapeutic genetic material or specific DNA sequences into a cell. In a viral vector, the viral genes are removed and replaced by a therapeutic DNA sequence encodes genes, RNAs or other substances and packaged inside the shell of a virus. The viral vector is then delivered directly to the body (in-vivo therapy) or to cells (ex-vivo therapy; see Gene-modified cell therapy below) to deliver the therapeutic genetic material to the nucleus of the cell.
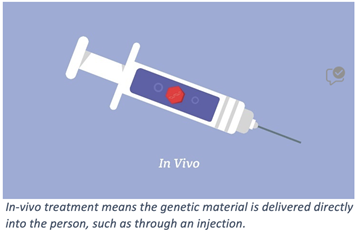 In-vivo viral vector therapies are frequently limited to a one-time delivery due to the innate immune response to the virus that usually prevents re-dosing. Learn more about innate immunity in Vectors 101. There are multiple FDA approved viral vector DNA therapies.
In-vivo viral vector therapies are frequently limited to a one-time delivery due to the innate immune response to the virus that usually prevents re-dosing. Learn more about innate immunity in Vectors 101. There are multiple FDA approved viral vector DNA therapies.
RNA therapy is the use of shorter sequences of genetic material in RNA format to treat or prevent a disease. There are many different types of RNA therapy because there are so many different types of RNA sequences that can affect cell functions. These types of therapies are delivered using viral vectors, or other non-viral vehicles such as lipid nanoparticles. They often need repeat dosing to maintain a therapeutic (good) effect since the DNA is not being altered or supplemented. Let’s explore the different types of RNA and corresponding therapies:
- Ribosomal RNA (rRNA) is theRNA that helps form ribosomes, which are the molecular machines used in building proteins.
- Messenger RNA (mRNA) is a middle message that can move through different parts of the cell to provide instructions to make proteins. It is a single stand that carries the information, initially stored within DNA, out of the nucleus to the cytoplasm of a cell where proteins are made.
- mRNA therapy is designed to produce more of a specific protein when the gene for that protein is missing, not working the way it should, or is beneficial for our bodies to create. Lipid nanoparticles may be used as containers to deliver therapeutic mRNA into cells because its structure protects the contents from being degraded after being injected into the body. Learn more about mRNA in vaccines.
- microRNA (miRNA) is a small form of single-strand RNA that typically targets multiple mRNAs to regulate expression of several different genes at the same time.
- miRNA therapies can be in the form of synthetic, double-stranded miRNAs (also called miRNA mimics), recombinant expression vectors that carry miRNA encoding sequences (naturally occurring or artificial), and oligonucleotide-based inhibitors (anti-miRs). Testing of miRNA therapies are ongoing in clinical trials.
- Small interfering RNA (siRNA) are double stranded RNA molecules that usually target a specific mRNA to prevent production of unwanted proteins.
- siRNA therapies are designed outside of the body to target expression of a specific gene. There are FDA approved siRNA therapies.
- Transfer RNA (tRNA) carry the building blocks of proteins, called amino acids, to the ribosome to help make a protein based off the mRNA instructions.
- Suppressor tRNA therapies are designed to override incorrect mRNA instructions that may cause disease by stopping protein production too early. Binding of suppressor tRNA to the incorrectly made mRNA allows the correct, full protein to be made by the ribosome. Suppressor tRNA therapies are still in the preclinical phase.
 Antisense oligonucleotides (ASO's) are synthetic (fake), single-stranded chains of molecules that target mRNA from a specific gene.
Antisense oligonucleotides (ASO's) are synthetic (fake), single-stranded chains of molecules that target mRNA from a specific gene.
- ASO therapies act inside a cell to alter how proteins are made. ASOs can silence a gene so a protein is not made. It can also alter how mRNA is made to then change how a protein is made. These stay in the cell for a limited duration and may need to be given repeatedly to maintain a therapeutic effect. There are multiple FDA approved ASO therapies.
- RNA aptamers are short pieces of RNA that bind to a specific protein to control their functions (for example, blocking or activating).
- Aptamer therapies are designed outside of the body. Unlike other RNA based therapeutics that must be brought inside a cell to be beneficial, RNA aptamers can tightly bind to proteins on the outside of the cell to provide unique therapeutic advantages. There are FDA approved RNA apatmers.
Cell therapy is the transfer of a specific cell type(s) into a patient to treat or prevent a disease. Depending on the cell therapy, the cells can come from either the affected individual or an unaffected donor. Some cell therapies are more common, like a hematopoietic stem cell (blood forming cells) transplant. Depending on the treatment, conditioning to prepare the body to receive the biological material is done to reduce the risk of an immune response and help the body successfully accept the cells. There are many FDA approved cell therapies.
- Gene-modified cell therapy (or ex vivo gene therapy) is a combination of gene and cell therapy. It first removes a person’s own cells from the body. Certain cell types are then treated by either adding a working or healthy copy of the gene or modifying/editing the affected one. The modified or treated cells are then returned to the person. Learn more about the FDA approved CAR T-cell Therapies for certain forms of cancers.
Next, explore how these approaches are used in various Disease Treatments. Learn more about the potentials, risks, and challenges of gene therapy.
Was this information helpful? If so, please share! All ASGCT Patient Education resources are free to use by sharing on social media, embedding the video, or simply linking to this page! Please credit the American Society of Gene and Cell Therapy or tag @ASGCTherapy.